Species diversity patterns of free-living marine nematodes in the
Aegean Sea
Nikolaos Lampadariou
Institute of Marine Biology of Crete,
P.O. Box 2214 710 03 Heraklion,
Crete, Greece
 |
Deep-sea nematode from the Aegean Sea
Nematodes are the most numerous multicellular animals on earth
inhabiting almost every possible environment. A handful of soil will
contain thousands of the microscopic worms, many of them parasites of
insects, plants or animals. Free-living marine forms are also very
abundant, including nematodes that feed on bacteria, fungi, and other
nematodes, yet the vast majority of species encountered are poorly
understood biologically.
Today, we can only speculate how many species of nematodes exist. For
example, there are estimates that they constitute up to 80% (Bongers,
1988) or 90% (Jairajpuri & Ahmad, 1992) of all metazoan on earth.
More recently, Lambshead (1993) estimated the number of nematode species
in the deep sea to as high as 1x108. It becomes evident that
if these estimations are pragmatic, then, we only know a tiny fraction
of this rich and successfully resistant to environmental degradation
taxon. Consequently, our interest lies more in what we can learn from
the nature itself since nematodes will probably be one of the last
groups that will disappear from the planet.
Over the last few years, the Institute of Marine Biology of Crete
(IMBC) has undertaken several studies on the diversity of free-living
marine nematodes in the eastern Mediterranean, thus fostering a better
understanding of local and regional biodiversity patterns.
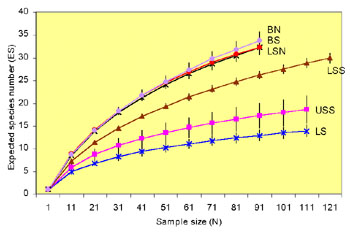 |
Fig. 1. Rarefraction curves for the different
zones of the Aegean Sea.
There is a long tradition in marine ecology to separate the
environment in different zones where certain assemblages occur, which
are different from those occurring in other zones. The benthic
environment is usually separated in zones according to two different
criteria, depth and latitude.
In the following analysis of diversity, the collected samples were
separated in six different zones: 1) littoral zone of the south
Aegean (LS), 2) upper sublittoral zone of the south Aegean (USS),
3) lower sublittoral zone of the south Aegean (LSS), 4)
bathyal zone of the south Aegean (BS), 5) lower sublittoral zone
of the north Aegean (LSN), and 6) bathyal zone of the north Aegean
(BN).
Data from the littoral and the upper sublittoral zone of the north
Aegean do not exist. In marine terminology, littoral is the tidal zone.
In areas such as the Mediterranean, where tides are practically
non-existent, the limits of the littoral zone correspond to the upper
and lower level of the swash zone. The sublittoral zone extends from the
lower watermark to 200 m depth whilst the bathyal zone extends from 200
m to 2,000 m. Deeper, the abyssal zone (2,000 to 6,000 m) and the hadal
zone (> 6,000 m) occur. However, these two zones do not exist in the
Aegean Sea.
In the analysis that follows, the sublittoral zone was further
separated in two sub-zones, the upper sublittoral (from the lower water
mark to 5 m depth) and the lower sublittoral (from 5 to 200 m depth). In
the Aegean, the upper sublittoral zone is generally considered as a
high-energy zone, in terms of water movements, whereas the lower
sublittoral is considered as a low-energy zone.
The analysis of nematode diversity in the Aegean Sea showed that
there was a definite bathymetric pattern, which was not linear. The
rarefaction curves (Sanders, 1968) for each zone are presented in Fig.
1. There is a gradual increase of diversity as depth increases. The
littoral zone (0 metres) shows the lowest diversity, whereas the highest
diversity values were found at the bathyal zone (200-2,000 m).
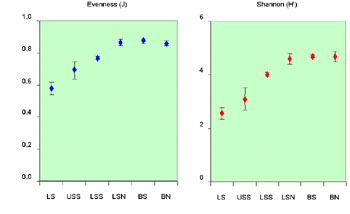 |
Fig. 2. Mean x standard errors for some
diversity indices for each zone.
Apart from the rarefaction curves, several other diversity indices
were calculated, both weighted for species richness and equitability. A
similar pattern of increasing diversity with depth is derived by these
indices (Fig. 2). These results were further supported by ANOVA, which
demonstrated that these differences were highly significant.
Nonlinear patterns of diversity have been found in several studies.
For example, Rex (1983) and Paterson et al. (1995), found
parabolic distribution patterns of macrobenthic diversity with pick
values near the bathyal zone in the Pacific and the Atlantic
respectively. Similarly, Boucher & Lambshead (1995), combining data
from the literature and Dinet & Vivier (1979), analysing data from
the Atlantic found parabolic diversity patterns for marine nematodes.
It appears, however, that macrofauna in the Aegean Sea does not
follow the above trend. As have been shown by Tselepides (1992) and
Karakassis & Eleftheriou (1997), the macrofauna diversity decreases
with depth, something they attributed to the decrease of food
availability with depth. Therefore, it appears that in the Aegean the
two benthic components show an inverse diversity response to
depth.
It would be very interesting to examine whether the observed increase
of nematode diversity in the Aegean follows the general parabolic
pattern found by others. The IMBC has recently started collecting
samples from several sites of the eastern Mediterranean around the 3,500
isobath, since the abyssal and hadal zones are absent from the Aegean
Sea. These samples are currently being analysed in order to test whether
the nematode diversity starts to decline after the bathyal zone.
Nevertheless, abyssal depth is the limit that can be reached for the
Mediterranean since no hadal environment exists in the entire basin.
Bibliography
Bongers, T. (1988). De Nematoden Van Nederland
Koninkliijke Nederlandse. Natuurhistorische Vereniging, Utrecht.
Boucher, G. & Lambshead, P.J.D. (1995).
Ecological biodiversity of marine nematodes in samples from temperate,
tropical and deep sea regions. Conservation Biology 9, 1594-1604.
Dinet, A. and M. H. Vivier (1979). Le meiobenthos
abyssal du golfe de Gascogne. II. Les peuplemets de nematodes et leur
diversite specifique. Cahiers de Biologie Marine 20: 109-123.
Jairajpuri, M.S. & Ahmad, W. (1992).
Dorylaimida. Free-living predaceous and plant-parasitic nematodes.
Oxford & IBH Publishing, New Delhi.
Karakassis, I. & Eleftheriou, A. (1997). The
continental shelf of Crete: structure of macrobenthic communities. Marine
Ecology Progress Series 160, 185-196.
Lambshead, P.J.D. (1993). Recent developments in
marine benthic biodiversity research. Oceanis 19, 5-24.
Paterson, G.L.J., Lambshead, P.J.D. & Gage,
J.D. (1995). Bathymetric patterns of polychaete diversity in the Rockall
Trough, north-east Atlantic. Deep-Sea Research 42, 1199-1214.
Rex, M.A. (1983). Geographic patterns of species
diversity in deep-sea benthos. In: G. T. Rowe Biology of the Pacific
Ocean depths. (pp. 453-472). New York: John Wiley & Sons.
Sanders, H.L. (1968). Marine benthic diversity: a
comparative study. American Naturalist 102, 243-282.
Tselepides, A. (1992). Ecological study of the
bathyal ecosystem of the Aegean Sea. PhD Thesis, Biology Department,
University of Crete, Heraklion.
|
